Innovate UK is the UK’s national innovation agency that supports business-led innovation projects. Businesses can apply for grants of up to £10m. Innovate UK grants are highly competitive, and to win, you need to invest significant time in preparing your application.
Myriad is the UK’s leading specialist R&D grant adviser. Our talented bid writing team boast some of the highest success rates in the UK and Europe.

We’ve created simple sections to help you understand how Innovate UK works and whether your business could be eligible for funding.
As the UK's innovation agency, Innovate UK is dedicated to powering growth through science and technology.
Through funding competitions open to all innovative businesses of any size or sector, they provide financial support that enables organisations to pursue R&D projects confidently and turn promising ideas into viable business opportunities - stimulating economic development across the nation.


Innovate UK is seeking projects that focus on research and development to generate new products, services, or processes.
When applying for a sector-based competition, ensure that your project or activity is eligible for the competition you’re entering, as each competition has different criteria.
Also, ensure all past Innovate UK-funded projects have been completed fully. They won't award funding if any previously awarded funding you've received has not been exploited. You will need to submit your application online using the Innovation Funding Service.

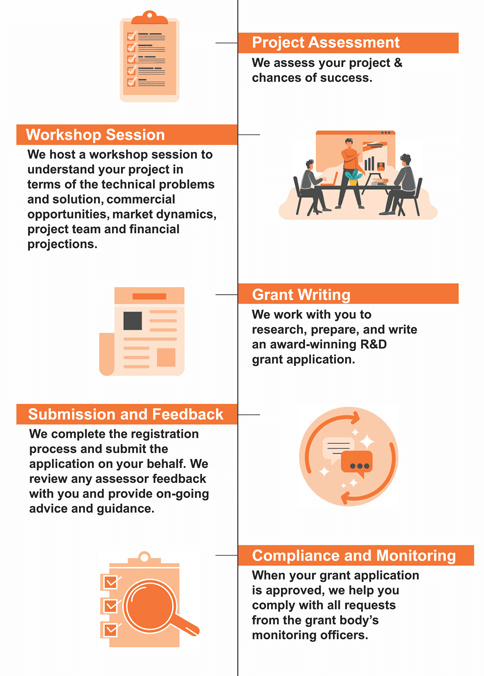
Investing in a grant application requires careful planning and attention to detail. Our experienced team can provide invaluable support, ensuring your proposal is crafted precisely to give you the best opportunity for success.
There are many pathways to gaining the necessary funding for your innovative project, and applying for a grant competition is one of them.
Before applying, ensure your proposal meets all requirements and qualifications the granting organisation outlines. Our experienced team can assess whether or not this opportunity aligns with what you're trying to achieve; don't hesitate to contact us if required!
Once submitted, each application undergoes an extensive evaluation process conducted by professional experts to determine its eligibility as a disruptive innovation capable of sparking success on both local and international levels.
"I have worked with Rehman on several grant proposals, and so far, he has kept us at a 100% success rate (approx. €4m to date!). Rehman can quickly get to the heart of deeply technical ideas and help translate them into proposals that win. He is driven and has excellent attention to detail, and I'd have no problem recommending him to anyone looking to do the same!"





Mark Brassil
Smart Reactors Ireland and Cerefuze Medical Devices UK Ltd
“As a result of working with Myriad, we have secured grant funding on three separate occasions, and we're currently waiting for the results of another grant application we've made. We certainly think that with Myriad’s help, we've been able to produce a very strong application.”





Chris Stanford
Arden Biotech
Myriad have an enviable track record with R&D grant applications. Using our grant application services can give you a significant competitive advantage over other grant applicants.
Our grant bid writers have secured over €200m for our clients in the last 24 months. We are also very proud to have secured some of the highest-scoring applications in Europe, with five of our projects scoring 15/15.
About Myriad

Projects should aim to lead to new products, processes or services (or novel use of existing ones) believed to be significantly ahead of anything similar in the field. They must focus on commercialisation, growth or scale-up.
The competition scope will specify the category of R&D activity for that particular funding opportunity.
Innovate UK supports the following R&D categories:
Each Innovate UK competition will have a different set of criteria your application must meet.
But there are a few general standards that all applications should adhere to.
Make sure the application does not:
The application will be rejected if it:
Has already been submitted for assessment in 2 other competitions.
If you are a UK-based business, you can apply for funding. All kinds of companies are eligible, from startups to large multi-national corporations.
If you are a UK-based business, you can apply for funding to:
Innovate UK focus their main activities on five strategic themes:
Learn more about these on the UKRI website: https://www.ukri.org/about-us/innovate-uk/our-plan-for-action/
Preparation of an Innovate UK application takes 150 to 300 hours of work.
You will need the right combination of people to cover the application's key aspects: the technology, market knowledge, commercialisation plan, and financial details.
Only applications that meet the funding competition’s eligibility criteria and scope are sent for assessment. You will be notified if your application is out of scope, with full reasons why.
Applications are assessed by up to five independent assessors, who judge the applications against the same scoring criteria. The assessors provide written feedback for each scored question.
All applications are judged on individual merit, with the Innovate UK funder’s panel making the final funding decision.
Some competitions are subject to a ‘portfolio’ approach to ensure that funds are allocated across the strategic areas of the competition’s scope. All successful applications must meet a quality threshold.
Your Innovate UK application is more likely to succeed if it:
Innovate UK applications are scored by up to five external assessors who are experts in the area of innovation identified in the application. These experts represent both business and research sectors.
Innovate UK aims to inform you whether or not your project will be funded around three months after the submission deadline.
Assessor feedback is provided on all applications. This is usually four weeks after you are notified of a decision.
For Innovate UK, project costs are only eligible if they are incurred and paid between the project start and end dates. These claims may be subject to an independent audit. Grants are claimed quarterly, in arrears, and are paid once the required audits and reports are complete.
Step 1 of #
Is your business registered for Corporation Tax in the UK or are you a partnership with corporate owners?
Have you developed new or improved existing products, processes or services in the last 2 accounting periods?
Does your business have fewer than 500 staff, and either: A turnover of no more than €100 million; or Gross assets of no more than €86 million?
Sorry, you must be a UK limited company or be a Partnership with corporate owners to be eligible for R&D tax credits.
In order to qualify for R&D tax credits you must be seeking to advance science or technology within your industry. As you’ve not developed any new or improved any existing innovative tools, products or services, and not re-developed any existing products, processes or services in the last 2 years. It is unlikely you have any qualifying activity. If you’re unsure, email or call us and we’ll help clarify.
In order to claim R&D tax credits, you need to either employ staff or spend money on contractors, consumable items and other items. If you’re unsure, email or call us and we’ll help clarify.
Thanks for that!
Congrats!! Based on your previous answers, you will qualify for the SME scheme. If you’d like some help maximising and securing your claim, please email or call us.
Congrats!! Based on your previous answers, you will qualify for the RDEC scheme. If you’d like some help maximising and securing your claim, please email or call us.
Speak to an expert Back